The majority of patients with GA want treatment to preserve their vision for longer3
In a claims analysis of newly diagnosed patients with GA,
~14%
of patients are being treated5*
Yet in a survey of people with self-reported GA,
84%
said they would try a treatment to slow their GA and preserve their vision for longer3†
GA requires a different mindset
It's about taking action now, so you can protect healthy retinal cells which may preserve vision as long as possible1,2
My goal as a physician is to preserve that vision for as long as I can, and I think it’s crucial to intervene early.”
– Dr. Arshad M. Khanani, Vitreoretinal Surgeon
I want to maintain my eyesight for as long as possible, and I felt a real sense of urgency to find a treatment that may help that.”
– Ken, real GA patient


Step into the role of protector today
When evaluating GA patients for treatment
Prioritize those at risk for accelerated progression1
The sooner you act, the more you may preserve for longer.1,2 See below for risk factors associated with faster progression of GA:
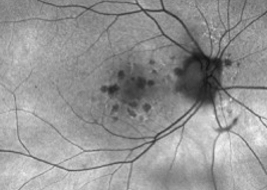
Multifocal lesions often grow faster than unifocal lesions6
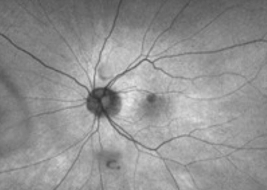
Extrafoveal lesions often progress faster than foveal lesions6
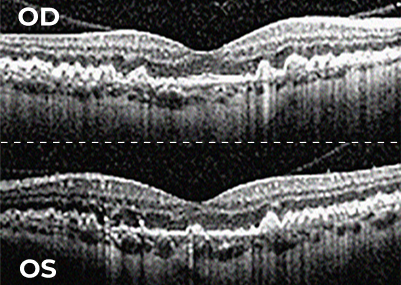
Patients with bilateral GA often progress faster than those with unilateral GA6,7
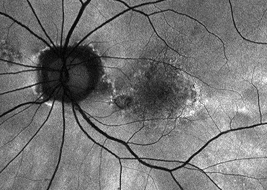
Hyperfluorescence on FAF imaging is often correlated with faster lesion progression6

Proactively protecting healthy retinal cells for longer is essential, especially in those at risk of faster progression. Patients appreciate this early management.1,2 It resonates with them if you are doing everything you can.”
– Dr. David S. Chin Yee, Retina Specialist
Quotes reflect patient experience at the time of printing. Physicians featured have been compensated.
FAF=fundus autofluorescence; GA=geographic atrophy; OD=oculus dexter; OS=oculus sinister.
*Data from Symphony Health, AMD Claims (142,301 newly diagnosed GA patients) June 1, 2020, to July 31, 2025.5
†Results from a telephone survey of 203 individuals with self-reported GA. Participants were compensated for their time, and the survey was sponsored by a pharmaceutical company.3
